The transfer of foreign genetic material from one organism to another is one of the pillars of modern-day genetic engineering, with applications from knocking out genes to protein production. However, there are several ways to transfer foreign nucleic acid into cells. Transformation, transfection, and transduction are different techniques for transferring genetic information and although they may sound similar, there are some key differences. Read on to learn about the distinctions between transformation, transfection & transduction and how these techniques are used in the lab.
What Is Plasmid Transformation?
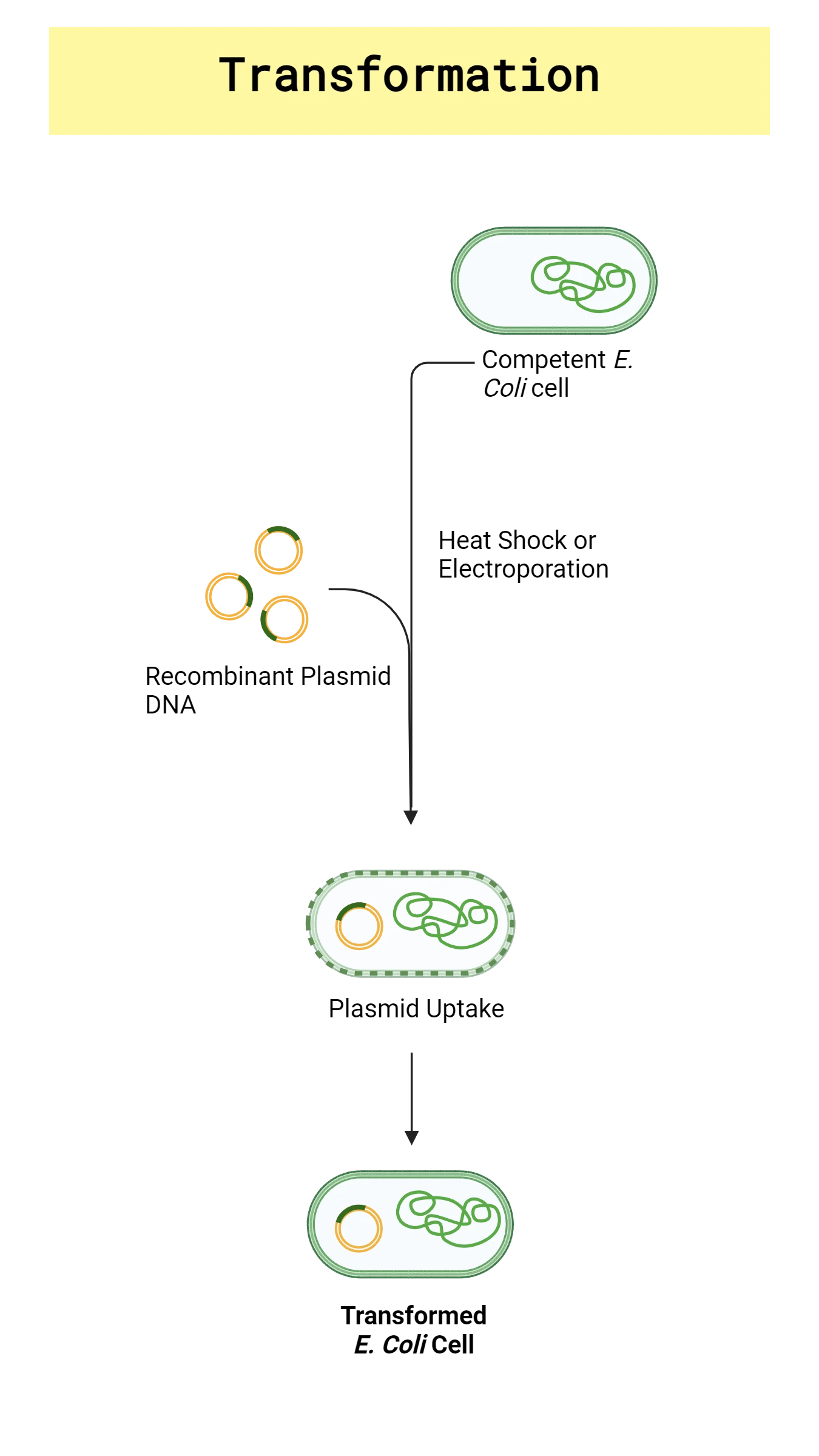
Transformation is the uptake of foreign DNA from the environment, such as plasmid DNA, typically by bacterial cells. Plasmid transformation is the transfer of plasmid DNA between organisms. Naturally occurring plasmids often confer benefits to the bacterial host, such as antibiotic-resistance or virulence, which facilitate their survival in a hostile environment. Scientists have harnessed the power of plasmid transformation to transfer recombinant plasmid into bacteria, yeast and even plant cells and tissue to express engineered DNA sequences in a variety of hosts.
Bacterial cells that are naturally able to take up foreign DNA without any manipulation are known as “naturally competent”. However, not all bacteria cells are naturally competent. For example, E. coli is widely used for transformations because it is easy to grow and well-studied, but E. coli must be made competent by using chemical or physical means. Zymo Research’s Mix & Go! E. coli Competent Cells are specially designed for simple, highly efficient transformations without the need for heat shock or lengthy incubation steps associated with standard procedures. E. coli plasmid transformations are regularly used to propagate recombinant plasmid in fast-growing bacteria for purification and further downstream analysis (Figure 1). Read our Introduction to Plasmid Blog for more information on plasmid structure and the necessary considerations for plasmid to be transformed into bacteria.
What Is Plasmid Transfection?
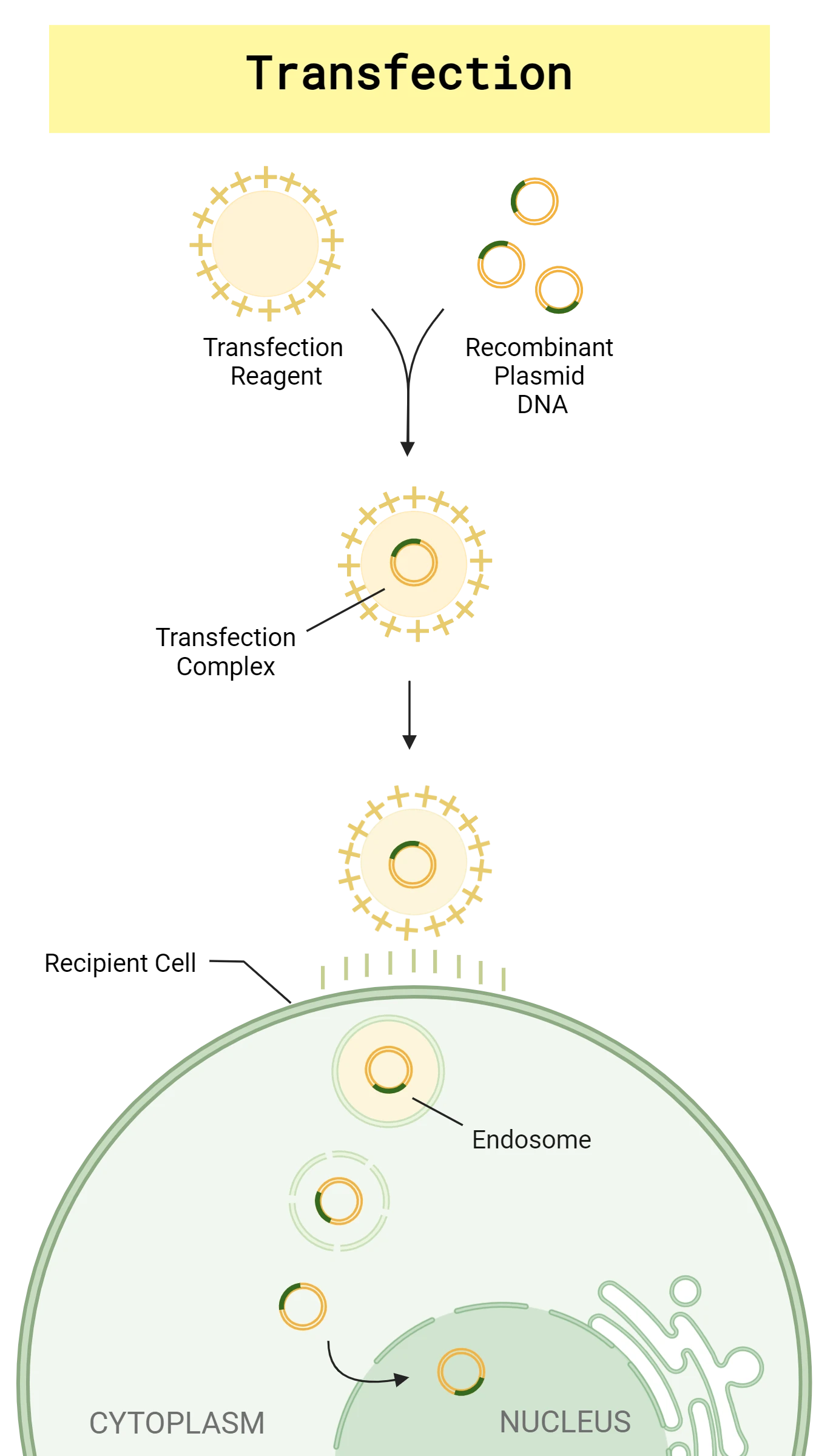
Transfection is when eukaryotic cells take up foreign DNA or RNA by non-viral means. Mammalian and many other eukaryotic cells do not naturally take up foreign nucleic acids, but scientists have discovered ways to introduce plasmid and other foreign DNA or RNA into eukaryotic cells. Much like the transformation methods for bacteria, there are both physical and chemical methods of transfection. One of the most common physical methods is electroporation, a technique in which an electrical pulse temporarily increases the cell’s permeability, facilitating uptake of foreign DNA or RNA. One of the major cons of electroporation is the potential for irreversible cell damage.1 Chemical transfection reagents are also commonly used and come in lipid and non-lipid varieties. Lipid-based transfection reagents will form positively charged nucleic acid containing liposomes that can integrate with the host membrane, enabling the entry of foreign nucleic acids, such as plasmid DNA1 (Figure 2). Different transfection methods have their pros and cons and should be selected based on the resources available, the type of cells to be transfected, and the nature of the nucleic acid cargo to be delivered.
Regardless of the method you choose, recovering endotoxin-free plasmid is critical to the success of your plasmid transfections. Endotoxins are present in the outer cell membrane of gram-negative bacteria, such as E. coli, and will elicit an immune response in mammalian hosts and cells if they are copurified with your plasmid. Some common transfection applications that require endotoxin-free plasmid include protein expression, CAR-T cell therapy, gene therapy and recombinant antibody therapy. Ensure that you recover concentrated, transfection-grade plasmid that is suitable for your intended downstream application.
What Is Transduction?
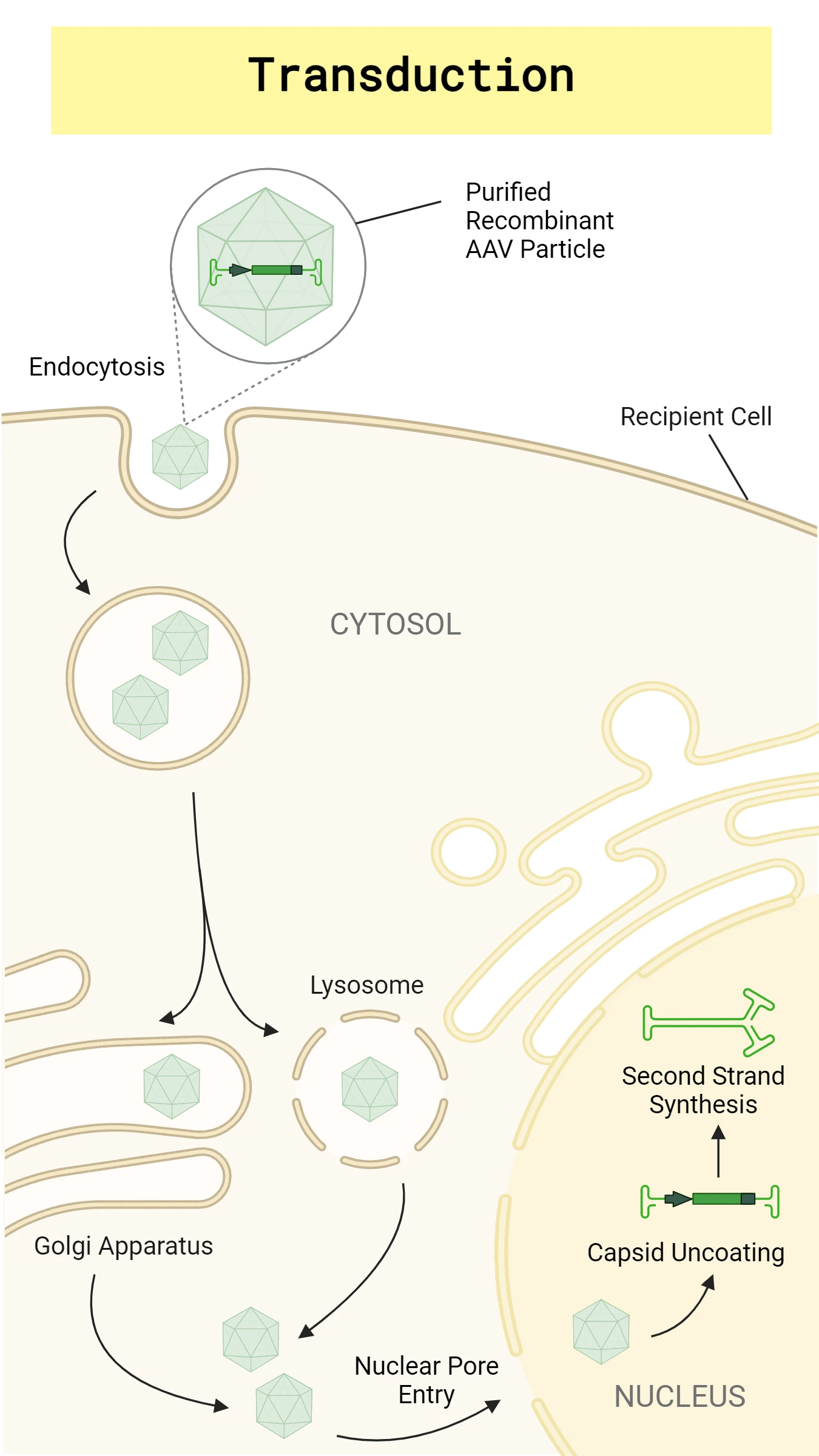
Scientists can modify viral nucleic acids to contain specific DNA sequences of interest. There are many different types of viruses that can be manipulated to introduce recombinant nucleic acids into different host cells. For example, bacteriophages introduce DNA or RNA into bacteria, whereas lentiviruses and adenoviruses are capable of infecting human cells. Viral transductions can be either stable or transient. Stable transductions are when the recombinant gene of interest is integrated into the host genome and constitutively expressed whereas transient transductions are when the gene of interest is not integrated into the host genome. Lentiviruses are often used for stable transductions whereas adenoviruses are typically used for transient transductions.1 Recombinant viral particles used for transduction are commonly produced by transfecting mammalian cells with specialized plasmids. See Figure 3 for the transduction pathway of adeno-associated virus (AAV), a ssDNA virus commonly used for transductions, in a mammalian cell.
To perform a transduction, you will need a host cell of interest (eukaryotic or prokaryotic) and a virus that can infect your chosen cell type. There are several points that must be considered when choosing either transductions or non-viral transfections, including virus strain growth and maintenance, risk of viral infection, size of recombinant DNA, the packaging of your DNA of interest into the viral particles, and the host cell. Despite the additional challenges to overcome, viral transduction is relied on as an effective method to transfect difficult cell lines both in vivo and in vitro and to observe long-term impacts of genetic alterations.1
Citations
- Chong, Z. X., Yeap, S. K., & Ho, W. Y. (2021) Transfection types, methods and strategies: a technical review. PeerJ, 9, e11165. .
- Berry, G. E., & Asokan, A. (2016) Cellular transduction mechanisms of adeno-associated viral vectors. Current opinion in virology, 21, 54–60.