In the vast and intricate microbial world, yeast plays an important role across a diversity of applications from fermentation in baking and winemaking to facilitating significant advancements in biotechnology. The yeast cell is a microscopic powerhouse, encased in a robust cell wall which must be broken down to release its cellular components in many applications. This review aims to highlight the complexities of yeast cell walls, analyze the difference between traditional mechanical lysis and enzymatic lysis, and illustrate the potential of Zymolyase Ultra across diverse scientific and industrial applications.
Understanding the Complexity of Yeast Cell Walls
Yeast, harnessed for food and beverage production for thousands of years, has experienced a resurgence of attention since the 19th century because of its diverse utility in biotechnology. Serving as a model organism for genomics research, metabolic engineering, cell biology, and synthetic biology, yeast also facilitates the production of a diverse range of valuable products, including metabolites, enzymes, and biopharmaceutical proteins such as insulin, vaccines, and human serum albumin. Breaking the tough cell wall of yeast is an essential step to investigating genetic processes, cell cycle regulation, signaling pathways, and other intracellular components. In medical and diagnostic contexts, breaking the yeast cell wall is critical for sensitive and accurate detection of pathogenic strains such as Candida species. However, lysing yeast cells is not a straightforward task due to the robust structure of the yeast cell wall. It comprises multiple components like β-1,3-glucan, β-1,6-glucan, chitin, and others. Additionally, it contains glycosylphosphatidylinositol (GPI) proteins, which are often heavily mannosylated and phosphorylated. The role of β-glucans is to provide rigidity, while glycoproteins influence adhesion, interaction, and cell wall permeability. Furthermore, chitin forms cross links with β-glucans, contributing to the overall strength of the cell wall.
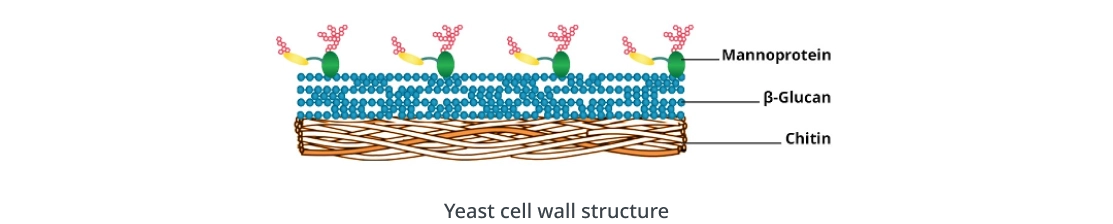
Structure of the yeast cell wall.
Mechanical vs. Enzymatic Lysis
Traditional methods of mechanical yeast lysis involve subjecting yeast cells to physical forces through techniques like bead beating, homogenization, or sonication. Mechanical lysis is not selective and can be applied to different sample types, including bacteria, yeast, plant cells and mammalian cells. However, due to the nature of mechanical lysis, it presents a spectrum of challenges:
- It often requires the use of specialized equipment, which can be costly to acquire and maintain.
- Mechanical lysis can be challenging to scale or automate.
- It is not selective and can result in contamination, compromising the extracted material.
- Mechanical lysis methods can subject yeast cells to shearing forces that may impact the integrity of sensitive intracellular components (nucleic acids and proteins), potentially affecting downstream analyses.
- Heat generated by mechanical lysis can lead to an increase in sample temperature, which may adversely affect the stability of certain biomolecules, such as RNA, proteins, or enzymes, and compromise their integrity and functionality.
- It can be labor-intensive and time-consuming. Achieving the right balance between efficient cell lysis and minimizing damage to biomolecules requires meticulous optimization of parameters and cooling steps.
In contrast, enzymatic lysis can provide a gentler and more precise solution. It presents a remarkably hassle-free alternative by eliminating the requirement for specialized instruments and fine tuning lysis conditions. With this method, cells are incubated with specially engineered enzymes such as Zymolyase (or Lyticase), which gently digests yeast cell walls without mechanical disruption. Furthermore, the reduced need for manual intervention in enzymatic yeast lysis makes it particularly appealing for high-throughput applications and large scale experiments, providing researchers and industries the ability to implement standardized cell lysis procedures across diverse settings.
While enzymatic yeast lysis methods are gentler and more specific compared to mechanical disruption, they also have their limitations:
- Many available lytic enzymes are not thorough in digesting yeast cell wall substances, leading to incomplete lysis.
- Most lytic enzymes are produced from microbial hosts, which can introduce contaminants like DNA and RNA into the enzymatic preparation. This is not ideal for use in bioburden-sensitive applications like microbiome profiling, next-generation sequencing (NGS), and PCR where the presence of these molecules can confound the analysis.
- Some enzymes require extended incubation times, leading to long turnaround times for the lysis step.
Enhanced Efficiency in Enzymatic Lysis
Zymolyase Ultra offers a simple solution to these challenges. Its distinctive formulation amalgamates multiple lytic activities including β-1,3-glucanase, β-1,6-glucanase and chitinase. The unique formulation ensures ultra efficient yeast lysis by targeting various yeast cell wall components more comprehensively than traditional enzymes. For example, 1 U of Zymolyase Ultra showed more efficient lysis of Candida albicans than 50 U of Lyticase.
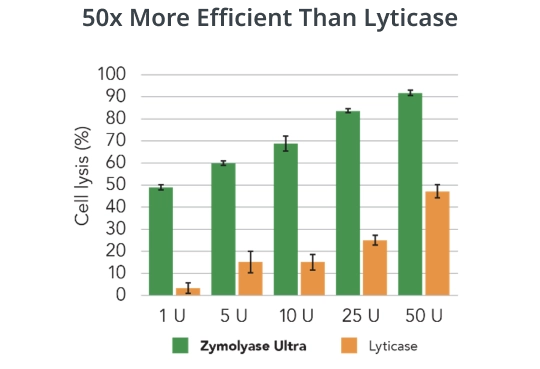
Compare Candida albicans lysis efficiency comparison between Zymolyase Ultra and Lyticase: 1 U Zymolyase Ultra is more efficient than 50 U Lyticase. 1-50 U Zymolyase Ultra or Lyticase were incubated with 8 x 107 C. albicans cells at 37°C for 30 min.
Minimized DNA Contamination in Enzymatic Lysis
Zymolyase Ultra ensures minimal DNA contamination and bioburden, a critical feature for various applications including pathogen detection by PCR and microbiome profiling by NGS. The ultra low DNA/RNA contamination is achieved by applying a unique bead surface chemistry into the purification process for the enzyme. This innovative approach ensures that the enzymatic lysis process with Zymolyase Ultra remains highly efficient while minimizing the risk of unwanted genetic material interference. In comparison to other common yeast lytic enzymes, Zymolyase Ultra exhibits up to 70-fold reduction in DNA contamination.
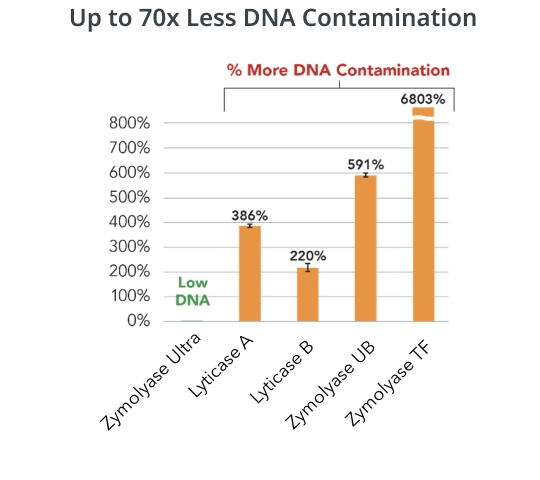
DNA contamination from bacteria and fungi was measured using the Zymo Research Femto Bacterial DNA Quantification Kit (E2006) and Femto Fungal DNA Quantification Kit (E2007), respectively. The amount of DNA was converted to genomic DNA copies/Unit enzyme in each assay and then combined.
Zymolyase Ultra Applications Across Industries
Zymolyase Ultra marks a significant breakthrough in yeast lysis approaches. Its diverse benefits not only unlock new possibilities but also reshape the landscape of research and industrial processes.
- Increasing Sensitivity in Infectious Yeast and Fungi Detection: By efficiently digesting the rigid cell walls of yeast and fungi, Zymolyase Ultra facilitates the release of nucleic acids, enabling more effective DNA/RNA extraction for sensitive analysis like PCR and qPCR. By improving fungal lysis efficiency, Zymolyase Ultra can enhance the sensitivity and accuracy of detection assays designed for oral, skin, nail, lung, urinary tract (UTI) and sexually transmitted (STI) infections.
- Plasmid, DNA, and RNA Extraction: Zymolyase Ultra increases nucleic acid extraction yield by significantly improving cell lysis efficiency. Thus, enzymatic lysis with Zymolyase Ultra reduces the overall lysis duration, thereby accelerating the entire workflow.
- Protein Extraction: Zymolyase Ultra enables efficient extraction of proteins from the cell wall, cell membrane, and intracellular compartments of yeast. Unlike mechanical lysis, enzymatic lysis protects sensitive proteins from denaturation caused by heating or physical shearing. This facilitates proteomic studies and downstream analyses such as protein-protein interaction assays, antibody based assays, Western blotting, and mass spectrometry.
- Microbiology Research and NGS: DNA contamination is a critical concern in microbiology experiments and NGS. With ultra low bioburden, Zymolyase Ultra ensures the extraction of clean and uncontaminated genetic material, minimizing the risk of introducing foreign DNA that could compromise the accuracy of NGS results.
- Cell Biology Research: Despite its simple unicellular structure, yeast shares fundamental cellular processes with higher eukaryotes, including humans. For this reason, researchers leverage yeast to explore fundamental cellular processes such as cell cycle regulation, DNA replication, gene expression and regulation, cell signaling pathways, metabolic pathways, aging, gene therapy, drug discovery, etc. With gentle enzymatic breakdown of yeast cell walls, cellular structures and components are protected from mechanical stress and damage, ensuring the integrity and functionality of the cellular components for downstream processes.
The applications Zymolyase Ultra can be applied to, including plasmid, DNA, RNA, and protein extraction, protoplast or spheroplast preparation, PCR detection, next-generation sequencing (NGS), cell biology research, and microbiology research.
In summary, Zymolyase Ultra is the culmination of innovative and dedicated research to overcoming the limitations of current yeast lysis methods. Its ability to efficiently lyse yeast cells while maintaining extremely low bioburden promises cleaner, more efficient, and more reliable results for scientists and bioprocess engineers in the fields of yeast detection, microbiome, cell biology, and synthetic biology.