A Sweet Solution to Obesity
Synthesizing the Anti-Obesity Agent Celastrol from Table Sugar
Obesity is a global health risk characterized by excessive fat deposits that affects 1 in 8 people around the world. Once considered a problem exclusively for high-income nations, obesity is on the rise globally due to poor diet and nutritional quality, with the disease incidence doubling in adults and quadrupling in adolescents since 1990. As of 2022, the World Health Organization reported that 890 million adults (aged 18 years or older) and 160 million children (aged 5 to 19 years) were living with obesity1.
The health consequences of obesity are severe, with obese individuals facing an increased risk of heart disease, type 2 diabetes, stroke, cancer, breathing difficulties, joint problems, gallbladder disease, and mental illnesses such as anxiety and depression2. The economic impact of obesity is staggering, as global costs associated with these conditions are projected to reach $3 trillion annually by 2030 and $18 trillion by 2060. This encompasses direct medical costs for diagnostic and treatment services as well as indirect costs such as sickness, premature death and disability, and decreased productivity.
Given these pressing issues, it is crucial to continue developing effective strategies and treatment options to manage obesity. While lifestyle changes such as dieting and increased physical activity are always recommended, certain cases require greater intervention using pharmacological medications or even bariatric surgery3. Thus, there is a global need for effective drug treatments to help combat obesity and support weight loss.
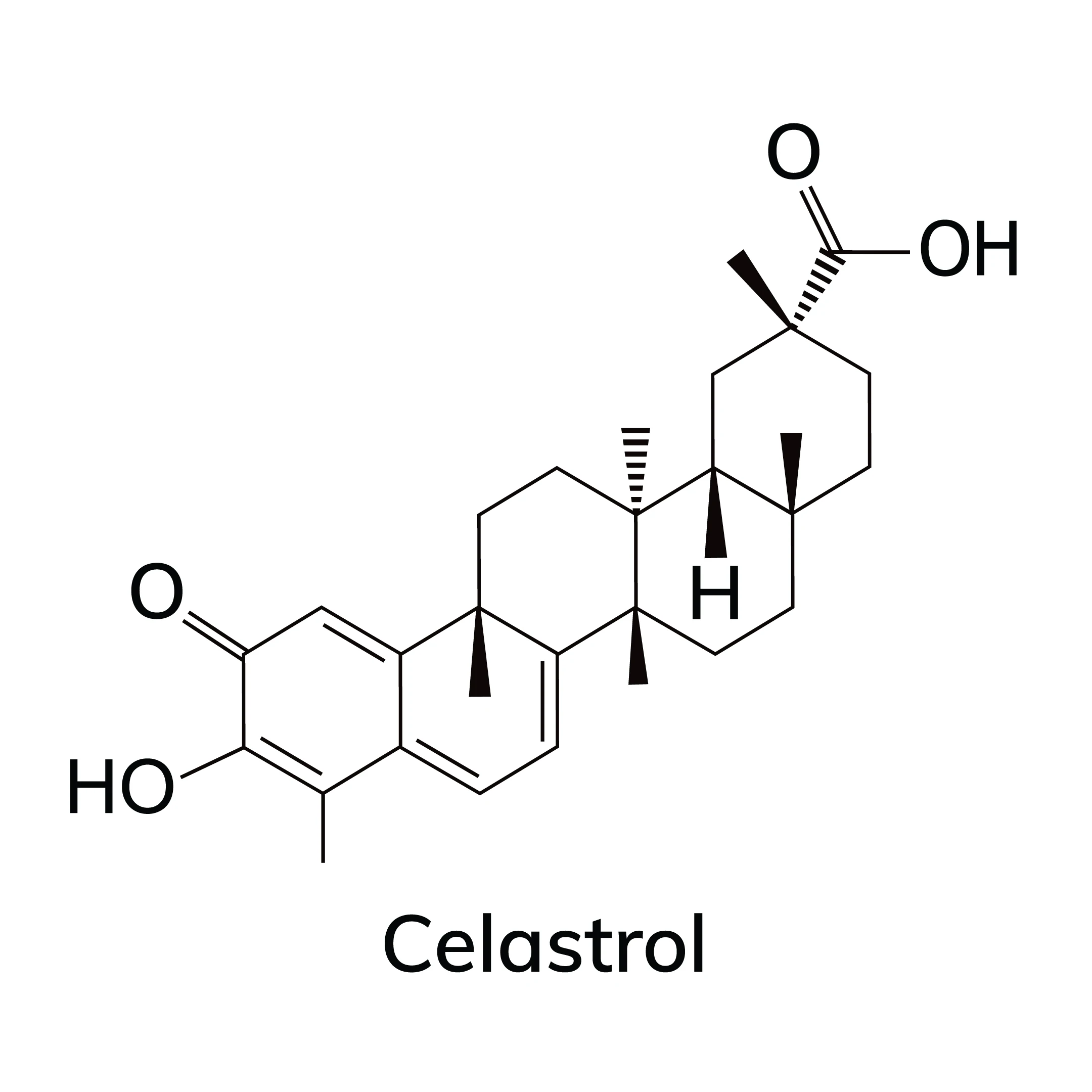
Celastrol is a pentacyclic triterpenoid found in the roots of Tripterygium wilfordii and has long been used to treat inflammatory disorders. Recently, it was discovered that celastrol could potentially be used to treat obesity, as it triggered substantial weight loss in obese mice4. However, harvesting enough celastrol from plant tissue to meet global demand is not feasible and its biosynthesis is not well documented. Recognizing these challenges, researchers with the University of Copenhagen conducted a study to characterize the celastrol synthesis pathway and develop a protocol for its scalable production5.
The scientists began their study by identifying transcripts corresponding to homologous cytochrome P450 enzymes that were overexpressed in root tissue of T. wilfordii. RNA transcripts were isolated, converted to cDNA, amplified by PCR, and then cloned for further characterization. Mach1 Escherichia coli cells were made highly competent in less than 45 minutes with Zymo Research’s Mix & Go! E. coli Transformation Kit and Buffer Set and then used for cloning. Plasmid constructs were purified from the cells, transformed into Agrobacterium tumefaciens, and then infiltrated into tobacco leaves for metabolite analysis.
Two cytochrome P450 enzymes exhibiting activity linked to the celastrol biosynthesis pathway were identified, enabling the researchers to rebuild the pathway in yeast cells and systematically define each intermediary compound. Saccharomyces cerevisiae cells were transformed, and digestion with the Not1 enzyme was used to release genes of interest from plasmids. YPD plates containing 5-Fluoroorotic Acid (5-FOA), a genetic counter-selection agent provided by Zymo Research, were used to easily screen for the correct yeast strains. The researchers successfully discovered the enzymes that oxidize friedelin, a precursor of celastrol, to celastrogenic acid. Celastrogenic acid is an intermediate that undergoes non-enzymatic conversion to celastrol. They also found that celastrogenic acid is spontaneously oxidized and decarboxylated, and then undergoes two cycles of catechol oxidation and double-bond rearrangement to form the quinone methide structure of celastrol.
Having confirmed the chemical pathway for celastrol synthesis, the researchers then focused their efforts on developing a scalable celastrol production system in yeast strains. The research group succeeded in developing an efficient synthesis method whereby table sugar was first converted to the key intermediate celastrogenic acid in an 8-step enzymatic pathway, and finally to celastrol with seven spontaneous steps split into two modules in engineered yeast.
This breakthrough method constitutes an easier, more scalable method for obtaining celastrol that previously could only be extracted from plant roots. Though the efficiency of this method must be improved before it can be commercially deployed, the synthetic production of celastrol starting from sugar in engineered yeast shows great promise. Given the previously demonstrated potent anti-obesity effects of celastrol, the scalable production of this compound could beget new medications with the potential to dramatically reduce the burden and health repercussions of obesity.
Explore products for therapeutic development and drug discovery
References
- Obesity and overweight. World Health Organization (2024). Obesity and overweight (who.int).
- Consequences of Obesity. Centers for Disease Control and Prevention (2022). Consequences of Obesity | Overweight & Obesity | CDC.
- Treatment for Overweight & Obesity. National Institute of Diabetes and Digestive and Kidney Diseases (2023).Treatment for Overweight & Obesity - NIDDK (nih.gov).
- Celastrol. Science Direct (2024). Celastrol - an overview | ScienceDirect Topics.
- Zhao, Y., Hansen, N. L., Duan, Y., Prasad, M., Motawia, M. S., Møller, B. L., Pateraki, I., Staerk, D., Bak, S., Miettinen, K., and Kampranis, S. C. (2023). Biosynthesis and biotechnological production of the anti-obesity agent celastrol. Nature Chemistry, 15, 1236-1246. https://doi.org/10.1038/s41557-023-01245-7.


