The emergence of new viral threats, such as H5N1 avian influenza, has raised alarms about the potential for pandemics. While much of the attention surrounding viral outbreaks tends to focus on the rapid spread and mutation of viruses, it is critical to highlight the ongoing development of tools and technologies that help prevent and contain these threats before they become widespread.
For over a decade, Zymo Research's DNA/RNA Shield™ sample transport technology has been instrumental in viral surveillance, supporting researchers and public health officials in detecting and responding to outbreaks. It has proven essential in preserving viral samples collected in remote areas, particularly for monitoring animal populations, such as poultry and bovine, for potential H5N1 outbreaks.
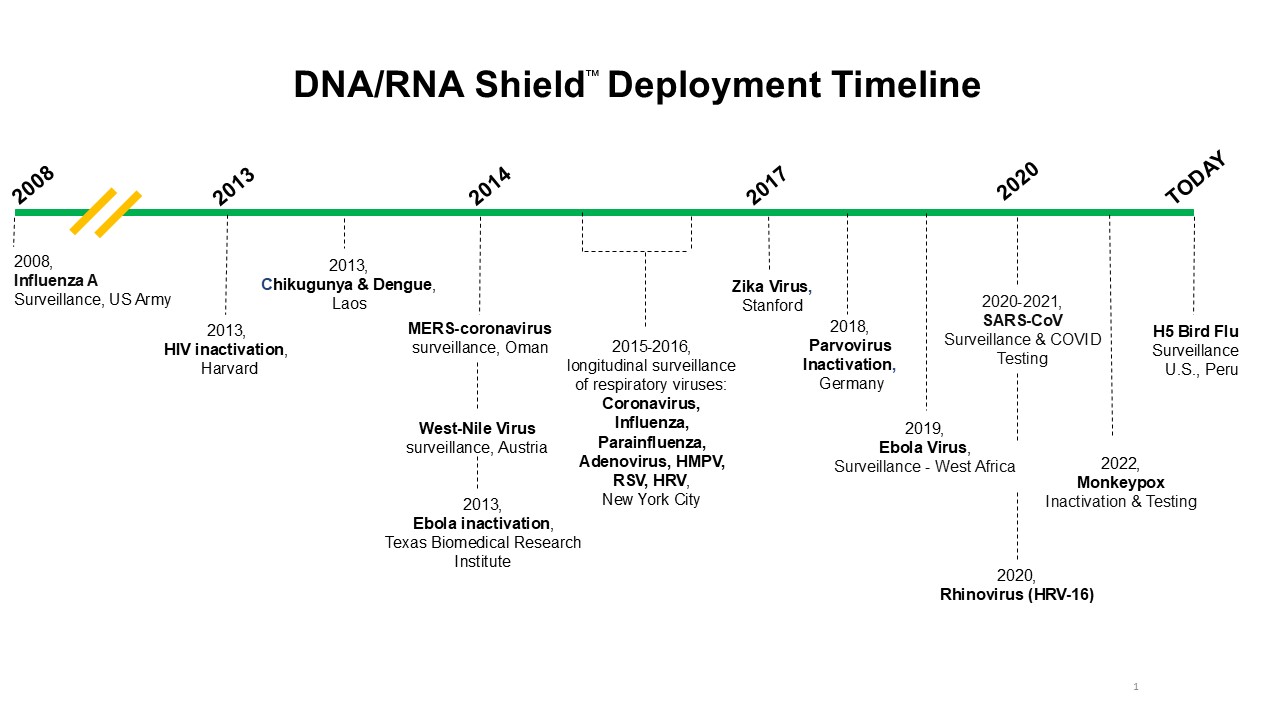
Below are key examples of how this technology has been applied in viral surveillance and its critical role in addressing the H5N1 threat.
Stabilizing Viral Samples for Reliable Detection
A major challenge in viral surveillance is maintaining the integrity of biological samples for accurate testing. The ability to stabilize RNA and DNA in collected samples, preventing degradation from environmental factors like temperature fluctuations and transport delays, is crucial. This technology has been used in global surveillance programs, including efforts by the U.S. Army in monitoring Influenza A and other viral pathogens. It is especially valuable in remote or resource-limited areas where cold storage is unavailable.
For H5N1, timely detection is essential, as the virus can spread rapidly among birds and sometimes jump to cattle or humans. Immediate preservation of samples allows for reliable surveillance and early detection, enabling action before the virus can cause widespread harm.
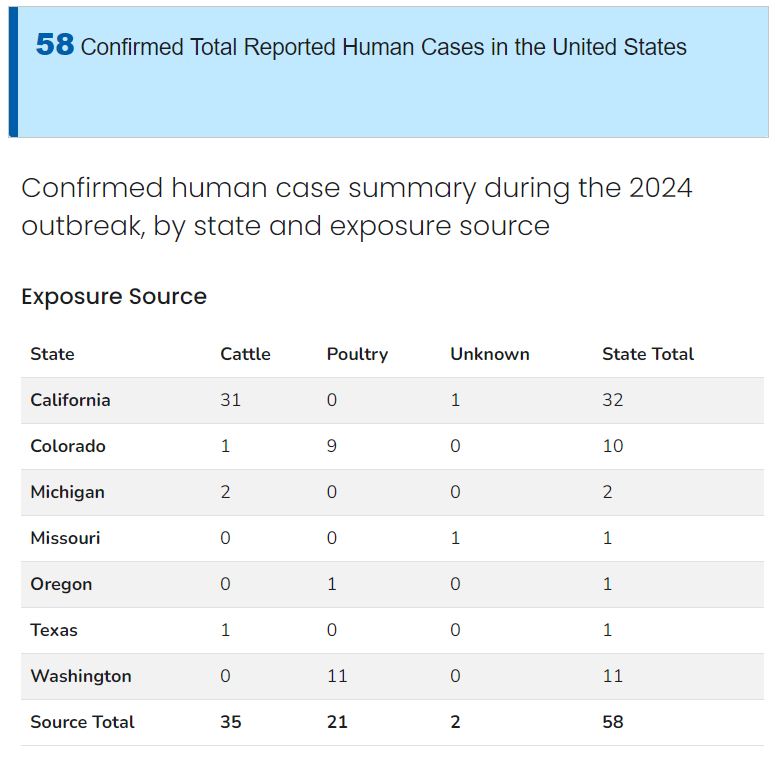
(Source: Center for Disease Control) Our home state of California currently holds the majority of confirmed human cases of H5N1 Bird Flu this year.
Enabling Longitudinal Surveillance of Avian Influenza
H5N1 is particularly concerning due to its potential for human-to-human transmission, though it primarily affects birds. Longitudinal surveillance is critical in tracking its spread, and preservative technologies have played a vital role in these efforts. For example, researchers at Columbia University utilized the technology for a longitudinal study on respiratory viral infections during the flu seasons over a 2 year span. This collection method was crucial for enabling consistent sampling across various sites with unpredictable environmental conditions.
Field Research and Rapid Response to Emerging Threats
Field research is often the first line of defense when viruses like H5N1 emerge in new areas. Researchers need to quickly collect samples from animal hosts and humans for testing. The preservative is designed to inactivate samples, ensuring they are safe for handling upon arrival at diagnostic labs for analysis. It has supported research on viruses where USDA APHIS permits are required, and 100% inactivation of viruses is an absolute requirement. In the case of zoonotic diseases like H5N1, rapid and safe sample collection is crucial for tracking mutations and shifts in transmission dynamics.

Veterinarian testing/vaccinating poultry
Enabling Rapid Diagnostic Testing
Accurate and timely testing is essential for effective outbreak response. Rapid diagnostic tests are critical for containment but require stable samples to produce reliable results. Through the COVID-19 Pandemic, DNA/RNA Shield was crucial in over 100 million COVID-19 tests, supporting accurate diagnostics and swift public health responses globally. This includes Zymo Research’s donating of over 1 million COVID tests to India and additionally to the Philippines. The technology ensures that RNA in samples remains intact, preventing degradation that could lead to false negatives or inconclusive results. The tested and proven technology could play a pivotal role in reliable testing is vital for H5N1 detection, and proper sample preservation ensures that collected samples remain viable for PCR testing and sequencing, even during transport.
Enhancing Our Response to H5 Bird Flu
As the global community continues to monitor H5N1 avian influenza, the lessons learned from past surveillance efforts and technologies are essential for protecting public health. The ability to stabilize, inactivate, and preserve viral samples enables accurate diagnostics, supports vaccine development, and strengthens global collaboration in viral surveillance.
In the event of an H5N1 outbreak, this technology will be vital for early detection, containment, and research, helping to prevent pandemics. With the right infrastructure, preparation, and tools, we can better protect public health and minimize the impact of emerging viral threats.
