Successfully Added to Cart
Customers also bought...
-
 DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the...
DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the... -
 DNA/RNA Shield SafeCollect Swab Collection Kit, 1ml (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA Shield completely inactivates harmful pathogens...
DNA/RNA Shield SafeCollect Swab Collection Kit, 1ml (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA Shield completely inactivates harmful pathogens...
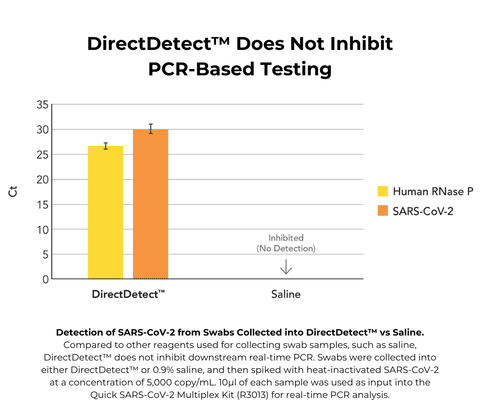
DNA/RNA Shield DirectDetect
| Cat # | Name | Size |
|---|
Highlights
- Eliminates the need for RNA (or DNA) extraction, allowing for rapid, direct, cost-effective analysis of samples
- No inhibition of real-time PCR
- Reduces sample viscosity to minimize pipetting errors with automated liquid handlers
Documents
Product Description
Technical Specifications
| Applicable For | RT-PCR, COVID-19 Testing |
|---|---|
| Reagent Storage | Ambient temperature => 1 year |
| Sample Collection | Swab (1 swab/mL), Saliva (50% v/v) |
| Sample Source | Saliva, oral (and nasal) specimen |
| Sample Stability | Ambient Temperature (4°C-25°C) < 1 week |
Resources
Q1: Can samples stored in DNA/RNA Shield be combined with DNA/RNA DirectDetect for bypassing sample extraction?
No, these are two separate reagents. Once a sample is collected in DNA/RNA Shield it cannot bypass extraction, it must be collected in DNA/RNA Shield DirectDetect in order to work as advertised.
Q2: Is DNA/RNA Shield DirectDetect the same as DNA/RNA Shield?
No, DNA/RNA Shield DirectDetect does not inactivate pathogens while DNA/RNA Shield does. Additionally, DNA/RNA Shield DirectDetect preserves nucleic acid for one week, while DNA/RNA Shield preserves RNA for at least 30 days and DNA for at least 2 years.
"In India, where testing supplies and vaccinations are difficult to obtain, testing is imperative to diagnosing the SARS-CoV-2 virus. While many biotechnology and testing suppliers have tended to concentrate on their domestic testing efforts, we appreciate Zymo Research's global humanitarian effort to help eradicate COVID-19."
Anu Acharya
Founder and CEO of Mapmygenome
"DNA/RNA Shield - DirectDetect enables countries seeing an increase in COVID-19 cases to perform COVID-19 testing without extraction; therefore, removing the barrier of limited extraction resources. It's our way of providing humanitarian support to people in need. We teamed up with Mapmygenome because of their ability to rapidly deploy and analyze RT-PCR testing."
Dr. Marc Van Eden
Vice President of Business Development at Zymo Research
Need help? Contact Us