Importance of COVID-19 Test Limit of Detection
Assay sensitivity must be weighed against other factors when selecting a COVID-19 testing platform
As the United States continues the effort to slow the spread of COVID-19, questions remain regarding the most effective testing strategies to implement. The widely accepted “gold standard” of COVID-19 testing utilizes real-time reverse transcription polymerase chain reaction (rRT-PCR), which has a very low limit of detection (LoD) 1. A lower limit of detection indicates greater analytic sensitivity, as the test can detect a positive result with fewer viral RNA copies per sample 2. However, there are trade-offs involving the limit of detection, the frequency and accessibility of testing, and the turn-around time required to generate results. Each of these factors must be weighed to develop a testing strategy that effectively limits the spread of COVID-19 in various communities.
What Is Limit of Detection?
The limit of detection is the lowest detectable concentration of viral RNA that returns a positive result in ≥95% of repeat measurements. Tests with a high limit of detection require more copies of viral RNA per sample to identify a positive case, resulting in a high proportion of false negative results for people who are at the beginning of an infection or are asymptomatic. The most compelling benefit of tests that have a low limit of detection, or high analytic sensitivity, is the ability to detect positive cases in an early stage of the infection, typically when the viral load is low. Early detection of SARS-CoV-2 positive cases allows infected people to quarantine before they become highly contagious.
RRT-PCR Tests
rRT-PCR tests, which amplify specific SARS-CoV-2 RNA targets and report a fluorescent signal, have a low limit of detection and are highly specific. The threshold cycle (Ct) at which SARS-CoV-2 targets become detectable during quantitative PCR can also provide clinically and epidemiologically relevant information regarding a patient’s viral load 1. The Ct value can help identify potential “super spreaders” who are most contagious to others. Additionally, rRT-PCR tests are compatible with high-throughput platforms, and hundreds of samples can be analyzed at once using 96-well or 384-well PCR plates.
Despite the ability to analyze many samples at once, the most notable drawback of using rRT-PCR based COVID-19 tests is the lengthy turn-around time. Sample collection, RNA extraction, and rRT-PCR can take around 2-4 hours, and patients typically do not receive their results for 24-48 hours after submitting a sample 3. The viral load often increases exponentially during early stages of the infection, so one day can make a huge difference in the infected individual’s ability to spread the illness 4.
Additionally, rRT-PCR tests require special equipment such as thermocyclers and must be performed in a laboratory by trained personnel, which makes them less readily available to patients and more expensive2. Sending patient samples to a laboratory for testing also requires the use of a transportation medium such as Zymo Research’s DNA/RNA Shield to preserve RNA integrity in the sample and inactivate any pathogens that are present, including SARS-CoV-2.
Rapid Antigen Tests
Rapid antigen tests, which detect protein markers on the outside of SARS-CoV-2, have the advantage of being fast, inexpensive, and easy to administer at the point of care 4. By avoiding RNA extraction and amplification steps, rapid antigen tests can typically deliver results in minutes rather than days and can be performed at a high frequency 4.
While rapid antigen tests have the advantage of a fast turn-around time, they lack the analytic sensitivity of rRT-PCR tests. The limit of detection (LoD) of rapid antigen tests is around 100-1000 times higher than most rRT-PCR tests, which means a greater number of true positive cases will be missed. It is estimated that “each 10-fold increase in LoD is expected to increase the false negative rate by 13%, missing an additional one in eight infected patients. The highest LoDs on the market will miss a majority of infected patients, with false negative rates as high as 70%” 1. The high fraction of false negative results reported by rapid antigen tests make it unlikely that this method alone can satisfy current public health needs 1.
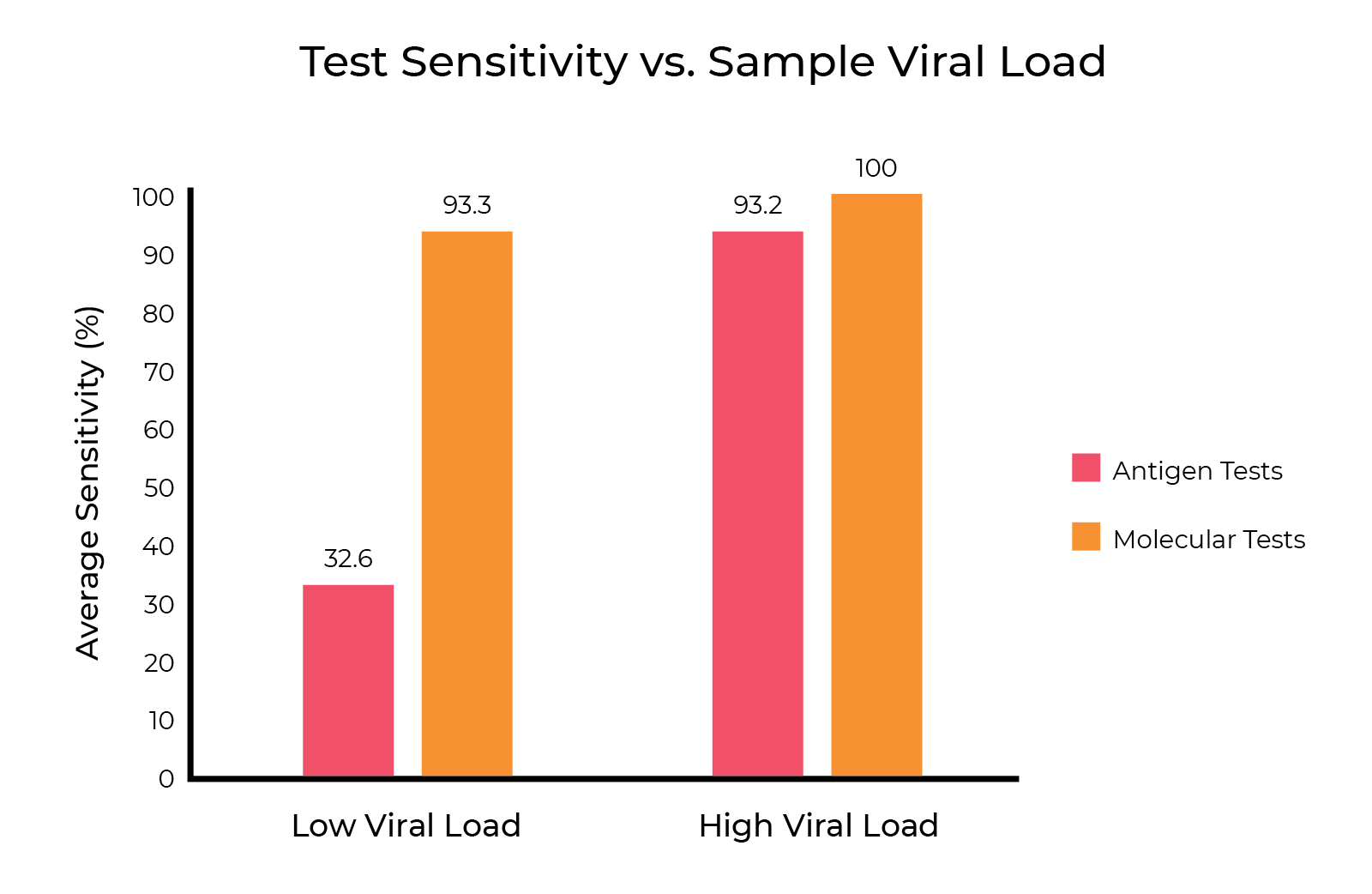
The delayed reporting of rRT-PCR results has the potential to cancel out the benefit of catching positive cases early, as individuals may enter a highly transmissible stage of infection before receiving their results and being instructed to self-isolate. Nevertheless, the high limit of detection of rapid antigen tests can result in false negative results for people who are asymptomatic or newly infected. People who receive a false negative result may choose not to self-isolate, which puts them at risk of spreading the infection as their viral load increases.
The Trade-off Between Speed and Sensitivity
The central goal of COVID-19 testing is to identify infected individuals in a highly sensitive and timely manner in order to stop the spread of the illness. Because there are benefits and drawbacks to both molecular and antigen-based COVID-19 tests, the best strategy to use often depends on the context and community in question.
If testing is needed for population surveillance services, it is best to select a strategy that can be implemented quickly and executed at a high frequency4. For example, a UC Berkeley model found that prioritizing quick turn-around time and testing frequency over test sensitivity (low limit of detection) prevented more cases in a surveillance effort of students and faculty5. This indicates that rapid antigen tests with same-day results may be the best option to prevent large outbreaks in an asymptomatic or pre-symptomatic community, as individuals with a high viral load and the potential to become “super spreaders” should be identified with the less sensitive test, quickly quarantined, and re-tested using rRT-PCR to confirm the results.
However, it is necessary to use a highly sensitive testing strategy in clinical settings, where patients must be given accurate diagnoses after a single test4. People who have symptoms or have been exposed to COVID-19 can make an informed decision to self-isolate before receiving their test results, which makes testing accuracy more important than speed to stop the spread of the illness in this case. rRT-PCR tests are the best fit to confirm a suspected COVID-19 infection, as they have the low limit of detection necessary to identify a positive result at various stages of the disease even when viral load is very low.
Releasing previously infected individuals from quarantine also requires careful consideration of testing options. Due to the low limit of detection needed to trigger a positive result using rRT-PCR tests, some people can continue to test positive for COVID-19 long after symptoms subside and they have passed the transmissible stage of the disease4. Using both rRT-PCR tests and serology tests, such as Zymo Research’s Quick COVID-19 Antibody Detection Kit, repeatedly to document patients’ recovery progress can allow for more informed decision-making on when to release people from quarantine, which in turn can support economic recovery from the pandemic3.
The importance of the limit of detection for COVID-19 is still being evaluated as more testing options become available. Speed of reporting must be weighed against test sensitivity, as measured by the limit of detection, to develop a COVID-19 testing strategy that can most effectively stop the spread of the disease and mitigate the effects of the ongoing pandemic.
Zymo Research’s Quick SARS-CoV-2 Multiplex Kit is our newest COVID-19 testing solution to achieve CE-IVD marking, and it has a very low limit of detection of 167 viral copies/ml (10 viral copies/reaction). Extracted RNA is added to a ready-to-use master mix that detects both SARS-CoV-2 and a human internal control during rRT-PCR, yielding accurate results in less than 2 hours.
Learn more about the Quick SARS-CoV-2 Multiplex Kit and its compatibility with high-throughput testing platforms:
Learn moreReferences:
1. Arnaout R, Lee RA, Lee GR, et al. SARS-CoV2 Testing: The Limit of Detection Matters. Preprint. bioRxiv. Published 2020 Jun 4. DOI: 10.1101/2020.06.02.131144.
2. Molecular-based Tests for COVID-19. Johns Hopkins Bloomberg School of Public Health, Center for Health Security. Published 2020 Nov 13. https://www.centerforhealthsecurity.org/resources/COVID-19/molecular-based-tests/
3. Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 surveillance. Preprint. medRxiv. Published 2020 Jun 27. DOI: 10.1101/2020.06.22.20136309.
4. Mina MJ, Parker R, Larremore DB. Rethinking Covid-19 Test Sensitivity — A Strategy for Containment. New England Journal of Medicine. Published 2020 Nov 24. DOI: 10.1056/NEJMp2025631.
5. Brook CE, Northrup GR, Ehrenberg AJ, et al. Optimizing COVID-19 control with asymptomatic surveillance testing in a university environment. Preprint. medRxiv. Published 2020 Nov 16. DOI: https://doi.org/10.1101/2020.11.12.20230870
6. Dinnes J, Deeks JJ, Adriano A, et al. Rapid, point‐of‐care antigen and molecular‐based tests for diagnosis of SARS‐CoV‐2 infection. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. DOI: 10.1002/14651858.CD013705.
7. Center for Devices and Radiological Health. SARS-CoV-2 Reference Panel Comparative Data. U.S. Food and Drug Administration. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-reference-panel-comparative-data


