Successfully Added to Cart
Customers also bought...
-
 DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the...
DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the... -
 DNA/RNA Shield SafeCollect Swab Collection Kit, 1ml (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA Shield completely inactivates harmful pathogens...
DNA/RNA Shield SafeCollect Swab Collection Kit, 1ml (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA Shield completely inactivates harmful pathogens...








ZymoPURE II Plasmid Maxiprep Kit












| Cat # | Name | Size | Price | Quantity |
|---|
Highlights
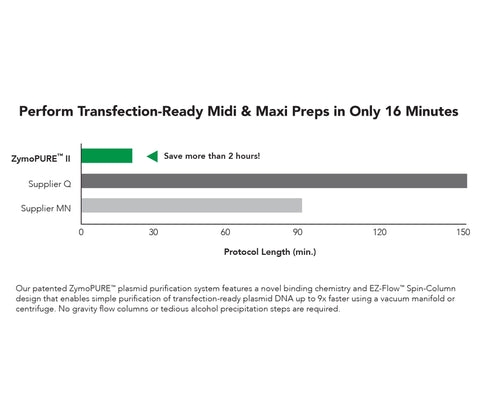
- Perform plasmid maxipreps in only 16 minutes using a simple spin-column protocol.
- Purify up to 3 mg of highly concentrated plasmid DNA directly from a spin-column.
- Eluted plasmid DNA is Endotoxin-free and Transfection-Ready.
Documents
Product Description
Technical Specifications
| Applicable For | Transfection, transformation, lentivirus production, adenovirus production, AAV production, CRISPR, genome editing, in vivo studies, sequencing, restriction endonuclease digestion, in vitro transcription/translation, PCR, and other sensitive applications. |
|---|---|
| Binding Capacity | 3.0 mg |
| Culture Input | ≤ 150 ml |
| Elution Volume | ≥ 300 µl |
| Endotoxin Levels | ≤ 0.025 EU/µg of Plasmid DNA |
| Equipment | Microcentrifuge and vacuum/vacuum manifold (recommended) or swinging bucket centrifuge. |
| Format | Spin-Column |
| Processing Time | ≤ 18 min |
| Purity | Typical Abs 260/280 ≥1.8 and Abs 260/230≥ 2.0 |
| Recommended Sample Volume | ≤ 150 ml |
| Size Range | Up to 200 kb |
| Yield | Up to 3.0 mg per preparation. Actual yield is dependent on the plasmid copy number, culture growth conditions, and strain of E. coli utilized. Typical yields from 150 ml of overnight culture grown in LB for high copy number plasmids are 600 – 1,200 µg. |
Resources
Q1: What is the composition of the ZymoPURE Elution buffer?
10 mM Tris-HCl, 0.1 mM EDTA, pH 8.5.
Q2: Can the ZymoPURE Kits be used with other bacteria?
Yes, the standard protocol should work with other gram-negative bacteria. However, the user will need to validate the protocol for their particular species. For gram-positive bacteria, please refer to the Gram-Positive Bacteria Protocol in the Appendix of the kit protocol.
Q3: What type of vacuum pump do you recommend?
The vacuum pump should be a single or double-staged unit capable of producing at least 400 mm Hg pressure at the vacuum manifold. If less pressure is applied, centrifuge the column prior to washing to remove any residual lysate/buffer remaining in the matrix.
Q4: Are the ZymoPURE kits compatible with any commercially available vacuum manifold?
Yes, any vacuum that uses standard luer-lock connectors is compatible.
Q5: Can an in-house vacuum line be used with the EZ-Vac Vacuum Manifold?
Yes, however, the pressure needs to be around 400 mm Hg for the liquid to pass through the columns quickly. Users should take caution, as the pressure of an in-house vacuum line can fluctuate drastically or be significantly reduced, depending on the demand in the building.
Q6: I ran out of ZymoPURE Wash 2. Can I substitute it with a homemade solution or Wash Buffer from another kit?
No, the ZymoPURE kits are only compatible with ZymoPURE Wash 2 and we cannot disclose a substitution due to the sensitive nature of the recipe. Additional ZymoPURE Wash 2 can be purchased separately.
Q7: Is there a protocol for low-copy number plasmid DNA?
Yes, the low-copy protocol can be found in the Appendix of the kit protocol.
Q8: Can I process more than the recommended volume of bacterial culture or use enriched growth media?
Yes, however, special care should be taken throughout the entire protocol since there is much more biomass. We recommend centrifuging the neutralized lysate before loading onto the supplied syringe filter. For further guidance on dealing with low-copy plasmids, please contact our technical support team. Exceeding the recommended volume for high copy plasmids can cause to overloading and subsequent clogging of columns, which can reduce DNA yield/quality or result in failed preps.
Q9: Can the ZymoPURE kits be used to isolate large plasmid constructs (BAC/PAC)?
Yes, the standard ZymoPURE protocol has been successfully tested with constructs up to 200 kb. To increase the elution efficiency of large plasmid DNA, we recommend pre-warming the ZymoPURE Elution Buffer (50 ºC) and increasing the incubation time on the column up to 10 minutes prior to centrifugation.
Q10: I accidently left my ZymoPURE P1 at room temperature, is it still okay to use?
Yes, the RNase A is fairly stable at room temperature, but we recommend placing it in 4 °C as soon as possible to ensure optimal performance throughout the life span of the product.
| Cat # | Name | Size | Price | |
|---|---|---|---|---|
| D4200-1-3 | ZymoPURE P1 (Red) | 3 ml | $11.30 | |
| D4200-1-13 | ZymoPURE P1 (Red) | 13 ml | $13.60 | |
| D4200-1-100 | ZymoPURE P1 (Red) | 100 ml | $40.80 | |
| D4200-1-150 | ZymoPURE P1 (Red) | 150 ml | $56.70 | |
| D4200-1-210 | ZymoPURE P1 (Red) | 210 ml | $70.20 | |
| D4200-1-410 | ZymoPURE P1 (Red) | 410 ml | $126.90 | |
| D4200-2-3 | ZymoPURE P2 (Blue) | 3 ml | $11.30 | |
| D4200-2-13 | ZymoPURE P2 (Blue) | 13 ml | $13.60 | |
| D4200-2-100 | ZymoPURE P2 (Blue) | 100 ml | $32.90 | |
| D4200-2-150 | ZymoPURE P2 (Blue) | 150 ml | $45.30 | |
| D4200-2-210 | ZymoPURE P2 (Blue) | 210 ml | $56.70 | |
| D4200-2-410 | ZymoPURE P2 (Blue) | 410 ml | $100.80 | |
| D4200-3-3 | ZymoPURE P3 (Yellow) | 3 ml | $11.30 | |
| D4200-3-13 | ZymoPURE P3 (Yellow) | 13 ml | $13.60 | |
| D4200-3-100 | ZymoPURE P3 (Yellow) | 100 ml | $32.90 | |
| D4200-3-150 | ZymoPURE P3 (Yellow) | 150 ml | $45.30 | |
| D4200-3-210 | ZymoPURE P3 (Yellow) | 210 ml | $56.70 | |
| D4200-3-410 | ZymoPURE P3 (Yellow) | 410 ml | $100.80 | |
| D4200-4-3 | ZymoPURE Binding Buffer | 3 ml | $11.30 | |
| D4200-4-14 | ZymoPURE Binding Buffer | 14 ml | $13.60 | |
| D4200-4-110 | ZymoPURE Binding Buffer | 110 ml | $44.20 | |
| D4200-4-150 | ZymoPURE Binding Buffer | 150 ml | $51.00 | |
| D4200-4-210 | ZymoPURE Binding Buffer | 210 ml | $63.40 | |
| D4200-4-410 | ZymoPURE Binding Buffer | 410 ml | $114.40 | |
| D4200-5-20 | ZymoPURE Wash 1 | 20 ml | $19.30 | |
| D4200-5-55 | ZymoPURE Wash 1 | 55 ml | $45.30 | |
| D4200-5-320 | ZymoPURE Wash 1 | 320 ml | $220.90 | |
| D4200-5-410 | ZymoPURE Wash 1 | 410 ml | $294.60 | |
| D4200-6-10 | ZymoPURE Wash 2 (Concentrate) | 10 ml | $20.40 | |
| D4200-6-12 | ZymoPURE Wash 2 (Concentrate) | 12 ml | $43.10 | |
| D4200-6-23 | ZymoPURE Wash 2 (Concentrate) | 23 ml | $45.30 | |
| D4200-6-28 | ZymoPURE Wash 2 (Concentrate) | 28 ml | $48.70 | |
| D4200-7-1 | ZymoPURE Elution Buffer | 1ml | $11.30 | |
| D4200-7-6 | ZymoPURE Elution Buffer | 6 ml | $12.50 | |
| D4200-7-12 | ZymoPURE Elution Buffer | 12 ml | $22.70 | |
| D4200-7-30 | ZymoPURE Elution Buffer | 30 ml | $43.10 | |
| C1001-50 | Collection Tubes | 50 Pack | $17.00 | |
| C1001-500 | Collection Tubes | 500 Pack | $58.90 | |
| C1001-1000 | Collection Tubes | 1000 Pack | $102.00 | |
| C1092-5 | ZymoPURE Syringe Filter-X | 5 Pack | $31.70 | |
| C1037-5 | ZymoPURE Syringe Plungers | 5 Pack | $15.90 | |
| C1082-5 | Zymo-Spin V-PX Column Assembly w/15 ml Reservoir-X and 50 ml Reservoir | 5 Pack | $97.40 | |
| C1051-5 | EndoZero Spin Columns | 5 Pack | $70.20 | |
| C1051-10 | EndoZero Spin Columns | 10 Pack | $108.80 | |
“Perfect! Finally, we got 600ug plasmid from 150ml E.coli culture in half an hour.”
- B. P., Los Angeles Biomedical Research Institute at Harbor-UCLA
“We were impressed by the high yield of plasmid DNA from this kit! From a 150 mL culture, I was able to isolate 0.912 mg DNA, in the same amount of time (and effort) I could get about half as much DNA from the comparable Qiagen kit.”
- C. S., Abbott Laboratories
“I was very satisfied with this product. It produced high DNA concentration eluting in 400 µl using the above mentioned cell line, which would generally only be obtainable with other kits eluting in much smaller volumes. This product is also miles ahead of other general Maxiprep kits in terms of time required for plasmid extraction.”
- J. P., University of Pretoria
Need help? Contact Us

